Background: Patients with Down syndrome (DS) have an approximately 10-fold increased risk of developing ALL, and the spectrum of genetic alterations differs from that of non-DS ALL. Rearrangement and/or overexpression of cytokine receptor-like factor 2 (CRLF2+) occurs in 50% of DS ALL, compared to only 5-10% CRLF2+ cases in non-DS children and adolescents. JAK2 or JAK1 mutations co-occur in about half of CRLF2+ cases in both DS and non-DS ALL. The prognostic significance of CRLF2+ ALL also appears to differ in the limited data reported to date, with less adverse impact in patients with DS compared to non-DS ALL. Here, we report the clinical characteristics and prognostic significance of B-ALL with CRLF2 overexpression and JAK alterations in children and adolescents/young adults (AYA) with DS who were treated on Children's Oncology Group (COG) clinical trials from 2003-2016.
Methods: We analyzed clinical, laboratory, and outcome data for 317 patients with DS B-ALL treated on standard risk (SR) trials AALL0331 and AALL0932 and high risk (HR) trials AALL0232 and AALL1131, for whom CRLF2 status and rearrangement partners (IGH or P2RY8) were ascertained by flow cytometric assessment of surface expression; fluorescence in situ hybridization; and/or polymerase chain reaction (PCR) testing. JAK mutations were ascertained in a subset by PCR and sequencing. Minimal residual disease (MRD) was assessed by flow cytometry at the end of induction (EOI) and at end of consolidation (EOC) for a subset of EOI MRD+ patients.
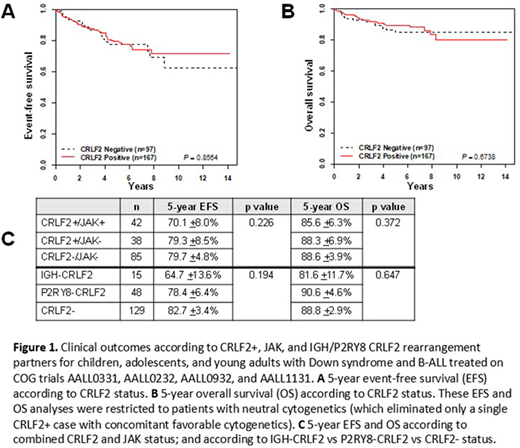
Results: We identified 168/317 (53.0%) CRLF2+ cases, and among those assessed for CRLF2 partner, 17/73 (23.3%) were IGH-CRLF2 and 56/73 (76.7%) were P2RY8-CRLF2. In the subset of 165 cases tested for JAK mutations (85 CRLF2- and 80 CRLF2+), 42/165 (25.4%) had JAK mutations, all of which co-occurred in CRLF2+ cases. CRLF2 positivity was significantly associated with younger age at diagnosis: 140/168 (83.3%) of CRLF2+ cases were under 10 years old, versus 106/149 (71.1%) of CRLF2- cases, p<0.01. P2RY8-CRLF2 cases were significantly more likely than IGH-CRLF2 cases to be associated with age under 10 years at diagnosis (92.9% vs 29.4%, p<0.0001), initial white blood count (WBC) <50x103 (80.4% vs 52.9%, p<0.024), National Cancer Institute (NCI) standard risk status (73.2% vs 23.5%, p<0.0002), and day 29 MRD <0.01% (67.9% vs 37.5%, p<0.028). There were no significant associations between JAK mutation status and any clinical features assessed (sex, initial WBC, central nervous system or testicular involvement, NCI risk group, or EOI/EOC MRD). Among patients with neutral cytogenetics (defined as neither favorable [ETV6-RUNX1, double trisomies of chromosomes 4 and 10] nor unfavorable [BCR-ABL1, KMT2A-rearranged, hypodiploid, iAMP21]), survival did not significantly differ between CRLF2+ and CRLF2- patients (Figure 1A,B), either for 5-year event-free survival (EFS) (79.1 +3.6% vs 77.6 +4.7%, p=0.856) or 5-year overall survival (OS) (89.5 ±2.8% vs 84.9 ±4.1%, p=0.674). There were also no significant differences in EFS or OS based on JAK mutation status or CRLF2 partner, although there were trends toward poorer outcomes in the CRLF2+/JAK+ and IGH-CRLF2 subgroups (Figure 1C). Finally, there was no significant difference in 5-year cumulative incidence of relapse (CIR) according to CRLF2 status (CRLF2+ 16.0 +3.0%, CRLF2- 10.6 +2.6%, p=0.179), although there were non-significant trends toward more relapses in CRLF2+ cases overall and in the NCI HR subgroup analysis.
Discussion: Whereas CRLF2 and JAK alterations are associated with higher MRD, poorer survival, and increased CIR in patients with high-risk ALL without DS, these alterations do not demonstrate strong adverse prognostic impact in children and AYAs with DS-ALL treated on recent frontline COG trials, although larger sample sizes are needed to adequately assess for possible poorer prognoses associated with the CRLF2+/JAK+ and IGH-CRLF2 subgroups. Regardless, given the frequency of these targetable lesions and the increased risk of relapse and chemotherapy-associated toxicities in patients with DS-ALL, targeted therapies currently under investigation for these genetic lesions may be beneficial to replace some intensive blocks of therapy in DS-ALL with CRLF2 and/or JAK alterations, both to enhance anti-leukemic efficacy and decrease intensive chemotherapy-associated toxicities.
Tasian:Gilead Sciences: Research Funding; Incyte Corporation: Research Funding; Aleta Biotherapeutics: Membership on an entity's Board of Directors or advisory committees. Borowitz:Amgen: Honoraria. Mullighan:Illumina: Consultancy, Honoraria, Speakers Bureau; AbbVie, Inc.: Research Funding; Pfizer: Honoraria, Research Funding, Speakers Bureau. Raetz:Celgene: Other: DSMB member; Pfizer: Other: Institutional research funding. Loh:Pfizer: Other: Institutional Research Funding; Medisix Therapeutics: Membership on an entity's Board of Directors or advisory committees. Hunger:Novartis: Consultancy; Amgen: Current equity holder in publicly-traded company, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal